
Outsourcing criteria within the Asia-Pacific
乔治临床( George Clinical ) (GC) conducted a brief survey on sponsors who are currently not a customer of GC. 30 companies were surveyed by GC’s Business Development Managers across six cou […]
了解更多
乔治临床( George Clinical ) (GC) conducted a brief survey on sponsors who are currently not a customer of GC. 30 companies were surveyed by GC’s Business Development Managers across six cou […]
了解更多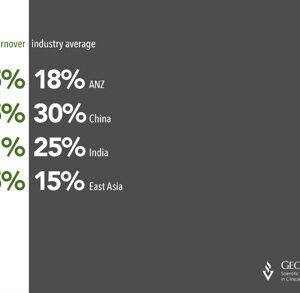
The cost of employee turnover in a Clinical Research Organisation (CRO) is high, as it weakens the relationship with the project sponsor. Research shows that in 2012, the CRO industry in the U […]
了解更多In a surprise move, the State Council in China has recently released a directive announcing its view on reforming the drug and medical device review and approval process in China. The State Co […]
了解更多乔治临床( George Clinical ) in China recently celebrated its second anniversary as an independent entity in China. Although 乔治临床( George Clinical ) has been conducting Phase 11 to Phase IV studies […]
了解更多China is a highly attractive clinical trial destination, and continues to see increased interest from CROs and drug development companies. China has a well-established tradition of developing medicines from basic research and the demands for modern pharmaceuticals is growing. Data gathered from clinicaltrials.gov indicated that in 2014, China had conducted 846 clinical trials, and an […]
了解更多乔治临床( George Clinical ) (GC) is at the heart of developing Asia Clinical Research Professionals. This summer, our Clinical and Regulatory Manager, East Asia volunteered as a trainer for Asia T […]
了解更多The Therapeutic Goods Administration (TGA) has released a new online form for the clinical trial notification (CTN) scheme. When a clinical trial is investigating the use of a product within Australia, which is not on the Australian Register of Therapeutic Goods (ARTG), or on the ARTG yet being used outside its marketing approval conditions, these […]
了解更多
The George Institute was recently profiled in The Lancet; revered as one of the most prestigious, peer reviewed medical journals in the world. The Lancet Vol. 385 April 18, 2015 The feature ar […]
了解更多
ARCS is Australia’s primary professional development association for people working in the development of therapeutic goods. At the annual scientific congress held in Sydney recently, Dr Maria […]
了解更多